Technologies
Our aim is to build a leading European CAR-T cell company and to make CAR-T therapies more accessible and affordable, which we also call to democratize CAR-T therapies for patients globally.
For this ambition, T-CURX is leveraging proprietary and cutting-edge technologies and CAR-T product opportunities circling around Sleeping Beauty transposon technology for scalable and straightforward CAR-T manufacturing developed in the laboratory of Prof. Hudecek. This cutting-edge research will be leveraged on one hand for the development of first- and best-in-class CAR-T cell product opportunities in both hematological and solid cancer indications and on the other hand to implement a highly scalable and affordable CAR-T manufacturing strategy. The commercial translation of T-CURX’ transformative CAR-T technologies and CAR-T products is led by T-CURX’ experienced leadership team headed by Dr. Ulf Grawunder as CEO. T-CURX' development is also supported by scientific key opinion leaders and Biotech-industry veterans with proven track records in developing innovative medicines and successful commercialization of biologiocal drugs in the fields of immunology and cancer.
T-CURX has been and is partner in a number of international consortia and projects, in which personalized, cellular immunotherapies are developed, including the Horizon-2020 EU translational project CARAMBA, the first clinical program in Europe validating T-CURX non-viral CAR-T manufacturing in a clinical setting.
T-CURX proprietary technologies include technology platforms addressing current limitations of CAR-T cell therapies: (i) a method for CAR-T cell engineering employing sleeping beauty transposon-based with innovative mRNA/minicircle DNA technology for higher scalability and reduction of COGS (see image below), (ii) a novel matchmaker technology allowing optimization of the CAR-spacer for optimal efficacy, and (iii) a novel liponano-particle delivery technology, by which the transposon-vector components are transferred to the patient's T cells via transposon-LNPs, enabling either highly scalable and patient centric bed-side CAR-T or eventually also in vivo CAR-T generation strategies.
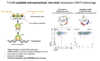
Relevant recent publications of T-CURX founders
-
CAR T cells targeting Aspergillus fumigatus are effective at treating invasive pulmonary aspergillosis in preclinical models
Sci Transl Med . 2022 Sep 28;14(664) doi: 10.1126/scitranslmed.abh1209. Epub 2022 Sep 28. -
Automated, scaled, transposon-based production of CAR T cells
J Immunother Cancer . 2022 Sep;10(9):e005189. doi: 10.1136/jitc-2022-005189. -
ATRA works synergistically with the γ-secretase inhibitor crenigacestat to augment BCMA on multiple myeloma and the efficacy of BCMA-CAR T-cells
Haematologica . 2022 Sep 1. doi: 10.3324/haematol.2021.281339 -
Highjacking myeloma's niche with beefed-up CAR-Ts
Blood . 2022 Jun 30;139(26):3671-3672. doi: 10.1182/blood.2022015707 -
Toward Rapid, Widely Available Autologous CAR-T Cell Therapy - Artificial Intelligence and Automation Enabling the Smart Manufacturing Hospital
Front Med (Lausanne) . 2022 Jun 6;9:913287. doi: 10.3389/fmed.2022.913287. eCollection 2022 -
Generation of CAR-T Cells with Sleeping Beauty Transposon Gene Transfer
Methods Mol Biol . 2022;2521:41-66. doi: 10.1007/978-1-0716-2441-8_3 -
Minicircles for CAR T Cell Production by Sleeping Beauty Transposition: A Technological Overview
Methods Mol Biol . 2022;2521:25-39. doi: 10.1007/978-1-0716-2441-8_2 -
The era of in vivo T cell engineering
Med (N Y) . 2022 Feb 11;3(2):85-86. doi: 10.1016/j.medj.2022.01.012 -
Time 2EVOLVE: predicting efficacy of engineered T-cells - how far is the bench from the bedside?
J Immunother Cancer . 2022 May;10(5):e003487. doi: 10.1136/jitc-2021-003487 -
Time to evolve: predicting engineered T cell-associated toxicity with next-generation models
J Immunother Cancer . 2022 May;10(5):e003486. doi: 10.1136/jitc-2021-003486 -
CART Initiatives in Europe
The EBMT/EHA CAR-T Cell Handbook [Internet]. Cham (CH): Springer; 2022. Chapter 5. 2022 Feb 7 -
Current Limitations and Perspectives of Chimeric Antigen Receptor-T-Cells in Acute Myeloid Leukemia
Cancers (Basel) . 2021 Dec 7;13(24):6157. doi: 10.3390/cancers13246157 -
T-Cell Metabolism in Graft Versus Host Disease
Front Immunol . 2021 Oct 29;12:760008. doi: 10.3389/fimmu.2021.760008. eCollection 2021 -
Siglec-6 is a novel target for CAR T-cell therapy in acute myeloid leukemia
Blood . 2021 Nov 11;138(19):1830-1842. doi: 10.1182/blood.2020009192 -
Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer
Nat Commun . 2021 Jul 1;12(1):4077. doi: 10.1038/s41467-021-24331-1 -
Actin cytoskeleton deregulation confers midostaurin resistance in FLT3-mutant acute myeloid leukemia
Commun Biol . 2021 Jun 25;4(1):799. doi: 10.1038/s42003-021-02215-w -
BTK inhibitors, irrespective of ITK inhibition, increase efficacy of a CD19/CD3-bispecific antibody in CLL
Blood . 2021 Nov 11;138(19):1843-1854. doi: 10.1182/blood.2020009686 -
Antibody-based cancer therapy
Oncogene . 2021 May;40(21):3655-3664. doi: 10.1038/s41388-021-01811-8 -
Composite CD79A/CD40 co-stimulatory endodomain enhances CD19CAR-T cell proliferation and survival
Mol Ther . 2021 Sep 1;29(9):2677-2690. doi: 10.1016/j.ymthe.2021.04.038 -
CARAMBA: a first-in-human clinical trial with SLAMF7 CAR-T cells prepared by virus-free Sleeping Beauty gene transfer to treat multiple myeloma
Gene Ther . 2021 Sep;28(9):560-571. doi: 10.1038/s41434-021-00254-w -
Immunocompetent cancer-on-chip models to assess immuno-oncology therapy
Adv Drug Deliv Rev . 2021 Jun;173:281-305. doi: 10.1016/j.addr.2021.03.015 -
Siglec-6 is a target for chimeric antigen receptor T-cell treatment of chronic lymphocytic leukemia
Leukemia . 2021 Sep;35(9):2581-2591. doi: 10.1038/s41375-021-01188-3 -
Homozygous BCMA gene deletion in response to anti-BCMA CAR T cells in a patient with multiple myeloma
Nat Med . 2021 Apr;27(4):616-619. doi: 10.1038/s41591-021-01245-5 -
Adoptive T cell immunotherapy for medullary thyroid carcinoma targeting GDNF family receptor alpha 4
Mol Ther Oncolytics . 2021 Jan 26;20:387-398. doi: 10.1016/j.omto.2021.01.012 -
Elotuzumab for the treatment of extramedullary myeloma: a retrospective analysis of clinical efficacy and SLAMF7 expression patterns
Ann Hematol . 2021 Jun;100(6):1537-1546. doi: 10.1007/s00277-021-04447-6 -
Toxicities of Chimeric Antigen Receptor T Cell Therapy in Multiple Myeloma: An Overview of Experience From Clinical Trials, Pathophysiology, and Management Strategies
Front Immunol . 2020 Dec 23;11:620312. doi: 10.3389/fimmu.2020.620312 -
Immunogenic Chemotherapy Enhances Recruitment of CAR-T Cells to Lung Tumors and Improves Antitumor Efficacy when Combined with Checkpoint Blockade
Cancer Cell . 2021 Feb 8;39(2):193-208.e10. doi: 10.1016/j.ccell.2020.11.005 -
CAR-T cells and TRUCKs that recognize an EBNA-3C-derived epitope presented on HLA-B*35 control Epstein-Barr virus-associated lymphoproliferation
J Immunother Cancer . 2020 Oct;8(2):e000736. doi: 10.1136/jitc-2020-000736 -
BATF3 programs CD8+ T cell memory
Nat Immunol . 2020 Nov;21(11):1397-1407. doi: 10.1038/s41590-020-0786-2 -
Immune-based Therapies for Hematological Malignancies: An Update by the EHA SWG on Immunotherapy of Hematological Malignancies
Hemasphere . 2020 Jun 29;4(4):e423. doi: 10.1097/HS9.0000000000000423 -
CAR T-Cells in Multiple Myeloma: State of the Art and Future Directions
Front Oncol . 2020 Jul 28;10:1243. doi: 10.3389/fonc.2020.01243 -
Overcoming key challenges in cancer immunotherapy with engineered T cells
Curr Opin Oncol . 2020 Sep;32(5):398-407. doi: 10.1097/CCO.0000000000000664 -
What is the future of immunotherapy in multiple myeloma?
Blood . 2020 Nov 26;136(22):2491-2497. doi: 10.1182/blood.2019004176 -
New targets and technologies for CAR-T cells
Curr Opin Oncol . 2020 Sep;32(5):510-517. doi: 10.1097/CCO.0000000000000653 -
Inefficient CAR-proximal signaling blunts antigen sensitivity
Nat Immunol . 2020 Aug;21(8):848-856. doi: 10.1038/s41590-020-0719-0. Epub 2020 Jul 6 -
Chemically Programmable and Switchable CAR-T Therapy
Angew Chem Int Ed Engl . 2020 Jul 13;59(29):12178-12185. doi: 10.1002/anie.202005432 -
Affinity maturation, humanization, and co-crystallization of a rabbit anti-human ROR2 monoclonal antibody for therapeutic applications
J Biol Chem . 2020 May 1;295(18):5995-6006. doi: 10.1074/jbc.RA120.012791 -
Antibody-Based CAR T Cells Produced by Lentiviral Transduction
Curr Protoc Immunol . 2020 Mar;128(1):e93. doi: 10.1002/cpim.93 -
A highly soluble Sleeping Beauty transposase improves control of gene insertion
Nat Biotechnol . 2019 Dec;37(12):1502-1512. doi: 10.1038/s41587-019-0291-z -
γ-Secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma
Blood . 2019 Nov 7;134(19):1585-1597. doi: 10.1182/blood.2019000050 -
Super-resolution microscopy reveals ultra-low CD19 expression on myeloma cells that triggers elimination by CD19 CAR-T
Nat Commun . 2019 Jul 17;10(1):3137. doi: 10.1038/s41467-019-10948-w -
The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells
Sci Transl Med . 2019 Jul 3;11(499):eaau5907. doi: 10.1126/scitranslmed.aau5907 -
Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplant
Bone Marrow Transplant . 2019 Nov;54(11):1868-1880. doi: 10.1038/s41409-019-0451-2 -
Cellular Therapy with Engineered T Cells, Efficacy and Side Effects
The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th edition. Cham (CH): Springer; 2019. Chapter 60 -
Clinical Utilization of Chimeric Antigen Receptor T Cells in B Cell Acute Lymphoblastic Leukemia: An Expert Opinion from the European Society for Blood and Marrow Transplantation and the American Society for Blood and Marrow Transplantation
Biol Blood Marrow Transplant . 2019 Mar;25(3):e76-e85. doi: 10.1016/j.bbmt.2018.12.068 -
Chimeric Antigen Receptor Library Screening Using a Novel NF-κB/NFAT Reporter Cell Platform
Mol Ther . 2019 Feb 6;27(2):287-299. doi: 10.1016/j.ymthe.2018.11.015 -
Novel Antibody Drug Conjugates Targeting Tumor-Associated Receptor Tyrosine Kinase ROR2 by Functional Screening of Fully Human Antibody Libraries Using Transpo-mAb Display on Progenitor B Cells
Front Immunol . 2018 Nov 2;9:2490. doi: 10.3389/fimmu.2018.02490 -
CAR T cells targeting αvβ3 integrin are effective against advanced cancer in preclinical models
Adv Cell Gene Ther . 2018 Sep;1(2):e11. doi: 10.1002/acg2.11 -
A Sortase A Programmable Phage Display Format for Improved Panning of Fab Antibody Libraries
J Mol Biol . 2018 Oct 19;430(21):4387-4400. doi: 10.1016/j.jmb.2018.09.003 -
Siglec-6 on Chronic Lymphocytic Leukemia Cells Is a Target for Post-Allogeneic Hematopoietic Stem Cell Transplantation Antibodies
Cancer Immunol Res . 2018 Sep;6(9):1008-1013. doi: 10.1158/2326-6066.CIR-18-0102 -
Non-viral therapeutic cell engineering with the Sleeping Beauty transposon system
Curr Opin Genet Dev . 2018 Oct;52:100-108. doi: 10.1016/j.gde.2018.06.003 -
CARs and other T cell therapies for MM: The clinical experience
Best Pract Res Clin Haematol . 2018 Jun;31(2):147-157 -
Novel targets and technologies for CAR-T cells in multiple myeloma and acute myeloid leukemia
Curr Res Transl Med . 2018 May;66(2):37-38. doi: 10.1016/j.retram.2018.03.006 -
CAR T-cells targeting FLT3 have potent activity against FLT3-ITD+ AML and act synergistically with the FLT3-inhibitor crenolanib
Leukemia . 2018 May;32(5):1168-1179. doi: 10.1038/s41375-018-0009-0 -
Current developments in immunotherapy in the treatment of multiple myeloma
Cancer . 2018 May 15;124(10):2075-2085. doi: 10.1002/cncr.31243 -
Expression of programmed death-1 on lymphocytes in myeloma patients is lowered during lenalidomide maintenance
Haematologica . 2018 Mar;103(3):e126-e129. doi: 10.3324/haematol.2017.178947 -
SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes
Blood . 2017 Dec 28;130(26):2838-2847. doi: 10.1182/blood-2017-04-778423 -
Mining Naïve Rabbit Antibody Repertoires by Phage Display for Monoclonal Antibodies of Therapeutic Utility
J Mol Biol . 2017 Sep 15;429(19):2954-2973. doi: 10.1016/j.jmb.2017.08.003
